Need Help? Bulk Orders? Ordering by P.O. number? Contact Customer Service for further assistance. Phone: 1-314-971-3028 | Email: [email protected]
Frequently Asked Questions
General Assay :
Is the room temperature important when incubating the plate in the final step before reading the fluorescence?
A: Yes, room temperature is important and should be about 25 °C. If the room temperature is below 20 °C , the assay performance will be affected.
What is the rotator mentioned in the protocol?
A: The rotator refers to a machine that rotates with different angles to allow media to move in each well. A shaker should also work as long as there is gentle shaking.
If the assay doesn’t work for me, do you offer technical support?
A: Yes, if you have any questions, you may ask us anytime at [email protected]. Out team is always glad to help !
Plate Reader and Microplate Settings:
What are the required settings for the plate reader to use the Bridge-It® Assay?
A: First, you would need a plate reader that is equipped for FRET (Fluorescence Resonance Energy Transfer). Then, you would need excitation filter at ~480-485 nm and emission filter at ~520 – 540 nm (for cAMP and cAMP-PDE Assays) and 665 nm (for other assays including SAM, L-Trp and L-Met). The plate should be read from top and plate reading settings of 50 times per well is recommended.
What are the required settings for the plate reader to use the FRET- PINCER® Assay?
A: First, you would need a plate reader that is equipped for FRET (Fluorescence Resonance Energy Transfer). Then, you would need excitation filter at ~485 nm and emission filter at ~540 nm (for donor signal) and 665 nm (for acceptor signal). The plates should be read from the top and plate reading settings of 50 times per well is recommended.
What are the required settings for the plate reader to use the TR-FRET Assays (TR-FRET Bridge-It® and TRF-PINCER®)?
A: First, you would need a plate reader that is equipped for TR- FRET (Time-Resolved Fluorescence Energy Transfer). Then, you would need excitation filter at ~330 nm and emission filter at ~620 nm (for donor signal) and ~665 nm (for acceptor signal). The plate should be read from top. Read settings at 50 times per well and delay time of 55 μs are recommended.
Do you have a suggested band pass for the excitation and emission filters?
A: Excitation and emission filters with varying bandpass are available and differs from vendor to vendor. However, we have been using the filter with following settings:
For TR-FRET based assays: (TR-FRET Bridge-It® and TRF-PINCER®):
330 nm excitation filter (band pass: 80 nm)
665 nm emission filter (band pass: 7.5 nm)
620 nm emission filter (band pass: 40 nm)
For FRET based assays: ( Bridge-It® and FRET-PINCER ®):
485 nm excitation filter (band pass: 20 nm)
665 nm emission filter (band pass: 34 nm)
540 nm emission filter (band pass: 40 nm)
What plate gain settings or sensitivity should be used?
A: Gain settings and sensitivity settings vary from one plate reader to other. Gain setting between 2,000-60,000 is fine.
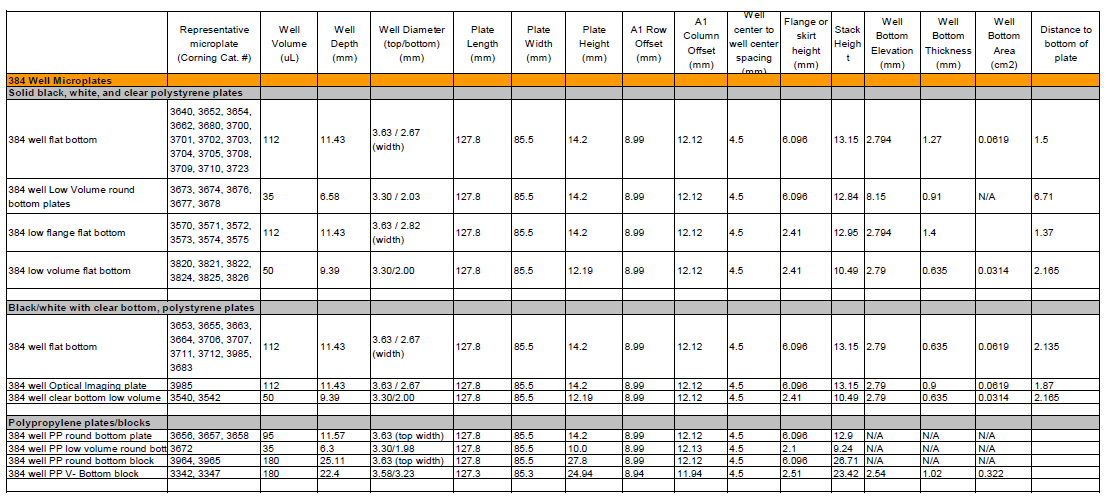
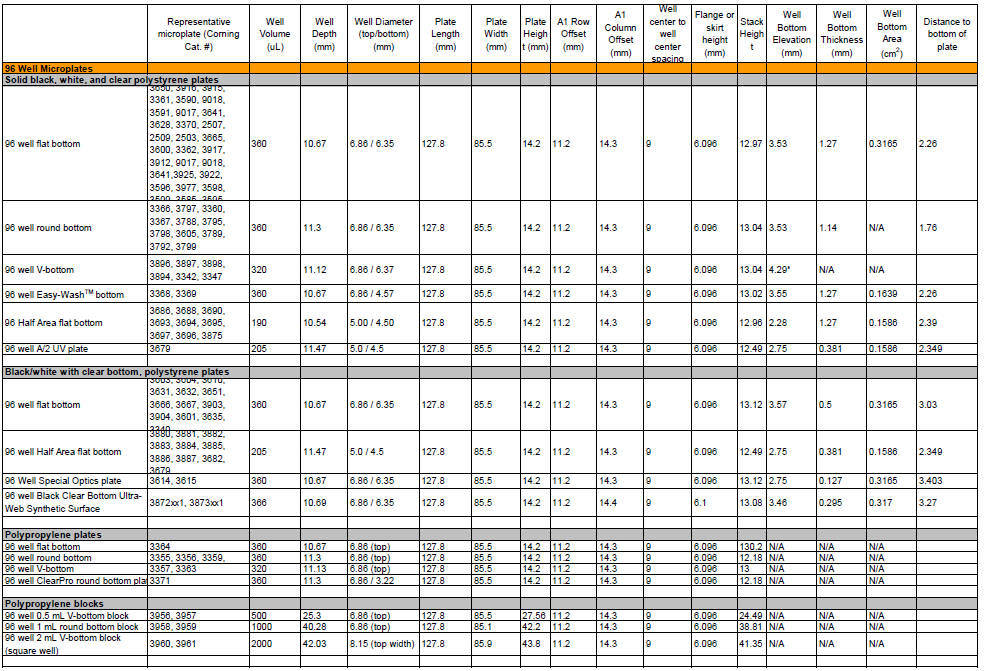
Does the type of microplate used to read the fluorescence matter?
A: Yes, you must use black non-binding surface plates to perform the assay, and read fluorescence from the top. A black non-binding surface microplate is recommended for following reasons:
-
Black walls reduce well-to-well crosstalk and autofluorescence during fluorescent assays
-
Non-binding surface creates a nonionic hydrophilic surface that minimizes molecular interactions with the plate surface