Frequently Asked Questions
General Assay
Is the room temperature important when incubating the plate in the final step before reading the fluorescence?
Yes, room temperature is important and should be about 25 °C. If the room temperature is below 20 °C , the assay performance will be affected.
What is the rotator mentioned in the protocol?
The rotator refers to a machine that rotates with different angles to allow media to move in each well. A shaker should also work as long as there is gentle shaking.
If the assay doesn’t work for me, do you offer technical support?
Plate Reader and Microplate Settings
What are the required settings for the plate reader to use the Bridge-It® Assay?
What are the required settings for the plate reader to use the FRET- PINCER® Assay?
What are the required settings for the plate reader to use the TR-FRET Assays (TR-FRET Bridge-It® and TRF-PINCER®)?
Do you have a suggested band pass for the excitation and emission filters?
Excitation and emission filters with varying bandpass are available and differs from vendor to vendor. However, we have been using the filter with following settings:
For TR-FRET based assays: (TR-FRET Bridge-It® and TRF-PINCER®):
330 nm excitation filter (band pass: 80 nm)
665 nm emission filter (band pass: 7.5 nm)
620 nm emission filter (band pass: 40 nm)
For FRET based assays: ( Bridge-It® and FRET-PINCER ®):
485 nm excitation filter (band pass: 20 nm)
665 nm emission filter (band pass: 34 nm)
540 nm emission filter (band pass: 40 nm)
What plate gain settings or sensitivity should be used?
Does the type of microplate used to read the fluorescence matter?
Yes, you must use black non-binding surface plates to perform the assay, and read fluorescence from the top. A black non-binding surface microplate is recommended for following reasons:
- Black walls reduce well-to-well crosstalk and autofluorescence during fluorescent assays
- Non-binding surface creates a nonionic hydrophilic surface that minimizes molecular interactions with the plate surface
One plate is already provided in the assay kit. If you need more microplates, you may buy either the 96-well (cat#: 163300) or 384-well (cat #: 163301) from us.
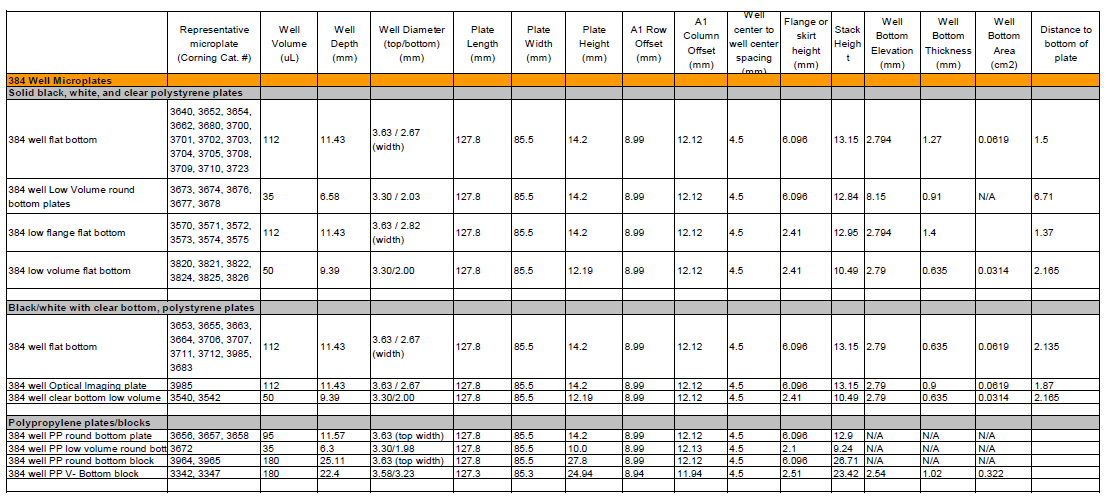
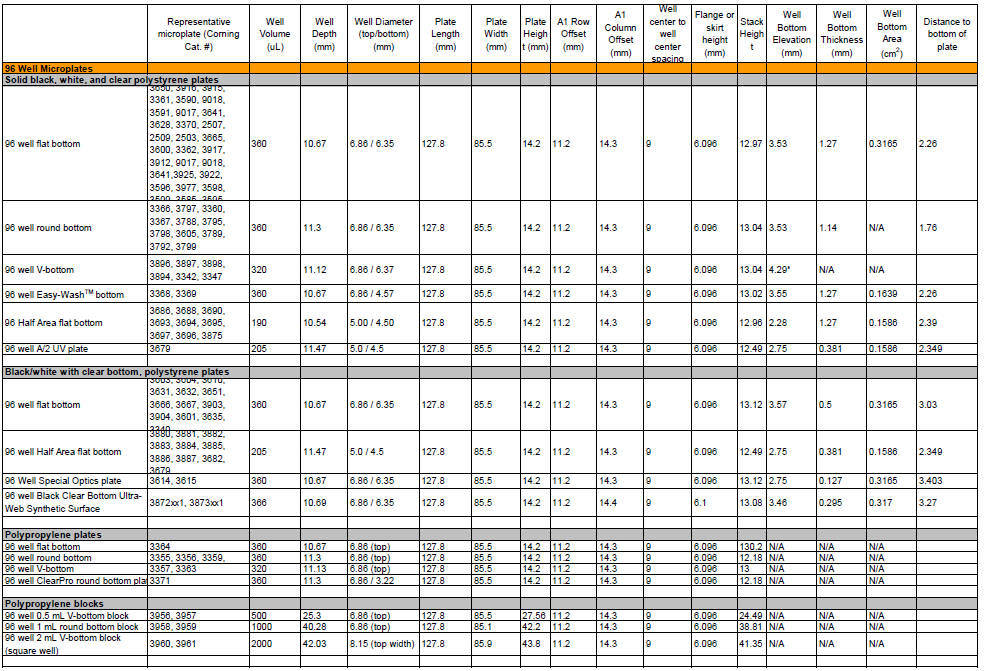
What are the plate dimensions for the plate that is provided in the kit?
The plate that is provided has the following dimensions shown in the images below. You can visit www.corning.com/lifesciences for additional information.