Human IgG TRF-PINCER Assay Kit, 384-well format
Mediomics’ human immunoglobulin G (hIgG) assay kit can be used to quantitatively detect the concentration of hIgG in biological samples. Product applications include drug and vaccine testing, high- throughput screening of hybridoma cell lines, as well as quality control and detection capabilities for antibodies in production or research programs. This kit is ideally suited for the rapid, simultaneous measurement of human IgG levels in large number of test samples due to its speed and simplicity. In comparison to the ELISA procedure, the TRF-PINCER® technology provides a simple (one mixing step) and fast (30 minute incubation) assay that is quantitative and reproducible.
$175.00 – $500.00
Description
This assay was developed for use in a 384-well plate format. A 96-well format is also available. Please inquire within.
Mediomics’ human immunoglobulin G (hIgG) assay kit can be used to quantitatively detect the concentration of hIgG in biological samples. Immunoglobulin G (IgG) is the most abundant immunoglobulin in human serum, and it is a biomarker of the adaptive immune response. Immunoglobulin levels can vary with health status, age, and drug treatment; therefore, quantitative measurements are a useful tool for pre-clinical researchers. Overabundance or, conversely, an absence of a certain specific subclass may be associated with disease.
Product applications:
1. Drug and vaccine testing to identify the presence/absence of an adaptive immune response.
2. High-throughput screening of hybridoma cell lines.
3. Continued monitoring of antibody production during manufacturing.
4. Maintenance of quality control guidelines for antibody lot production.
5. Rapid detection of IgG antibodies in a pull-down or co-IP assay.
6. Detection of hIgG concentration within biological samples as part of a pre-clinical research program.
Product Advantage:
This kit is a unique, simple, and fast tool for high throughput screening for the best hybridoma clones during the development of humanized monoclonal antibodies. Undiluted samples can be used to quickly and easily monitor the production of human IgG class polyclonal and monoclonal antibodies during pre-clinical development and manufacturing.
Mediomics’ hIgG TRF-PINCER® assay is ideally suited for the rapid, simultaneous measurement of human IgG levels in large number of test samples due to its speed and simplicity. It is readily adaptable to the high-throughput screening platforms currently being used in drug discovery research.
In comparison to the ELISA procedure, the TRF-PINCER® technology provides a simple (one mixing step) and fast (30 minute incubation) assay that is quantitative and reproducible.
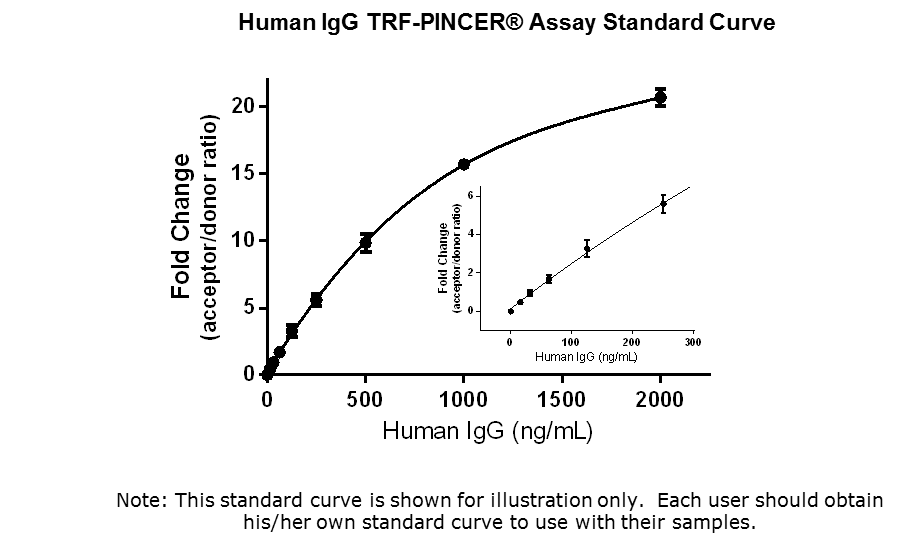
Detection Range: 15.6 – 2000 ng/mL
Limit of Detection (LOD): 15.6 ng/mL
CV value: < 5%
Pricing: The 100-well kit is a one-time trial purchase. For bulk pricing options, please contact us. For any other questions or comments, please do not hesitate to contact us. We are always happy to help!
Please refer to our technology pages for information on the scientific principles and technological advancements of the TRF-PINCER® assays.
-
PINCER® is a registered trademark of Mediomics, LLC, St. Louis, Missouri, U.S.A. Mediomics has a worldwide, exclusive license for this assay platform from Saint Louis University, St. Louis, Missouri.
-
The TRF-PINCER® Assay kit is provided for laboratory R&D use only. This product is not approved by the U.S. Government or by the government of any other country of the world for use in the clinical diagnosis of disease, or treatment of disease in humans or animals.
-
Patents allowed and pending in the U.S. and abroad.
-
PINCER® products have been developed with support, in part, from the National Institutes of Health, through STTR Phase I and Phase II Grants.