Human Albumin TRF-PINCER Assay Kit, 384 well format
The human Albumin TRF-PINCER Assay is ideally suited for screening and quantification of human albumin in urine, saliva, culture media, plasma, and serum samples. Serum albumin is important in regulating blood volume by maintaining oncotic pressure. Low serum levels are associated with liver damage/cirrhosis of liver, nephritic syndrome, inflammation and malnutrition. High albumin levels usually reflect dehydration. The TRF-PINCER® assay is an easy and flexible way to get fast, sensitive, and consistent results.
$175.00 – $500.00
Description
This assay was developed for use in a 384-well plate format. A 96-well format is also available. Please inquire within.
This assay is ideal for quick screening and quantification of human albumin in urine, saliva, culture media, plasma, and serum samples.
Human serum albumin (HSA), 67 kDa, is a non-glycosylated, negatively charged plasma protein synthesized primarily in the liver. Albumin comprises approximately one-half of the blood serum protein. Serum albumin is important in regulating blood volume by maintaining oncotic pressure. It effectively buffers plasma pH. Albumin also serves as a carrier of hydrophobic molecules, including lipid soluble hormones, bilirubin, bile, calcium, and iron. Several low- and high-affinity ligand binding sites on HSA are responsible for the binding of most pharmaceutical drugs. Clinically, serum albumin measurements are used as a diagnostic screen for several disease conditions. Low serum levels are associated with liver damage/cirrhosis of liver, nephritic syndrome, inflammation and malnutrition. High albumin levels usually reflect dehydration.
Product Applications:
-
Determine protein concentration of biological samples
-
High-throughput screening of fusion proteins used in drug delivery
-
Biomarker of kidney or liver damage, malnutrition, dehydration
The protocol for the TRF-PINCER® Assay is simple and fast.
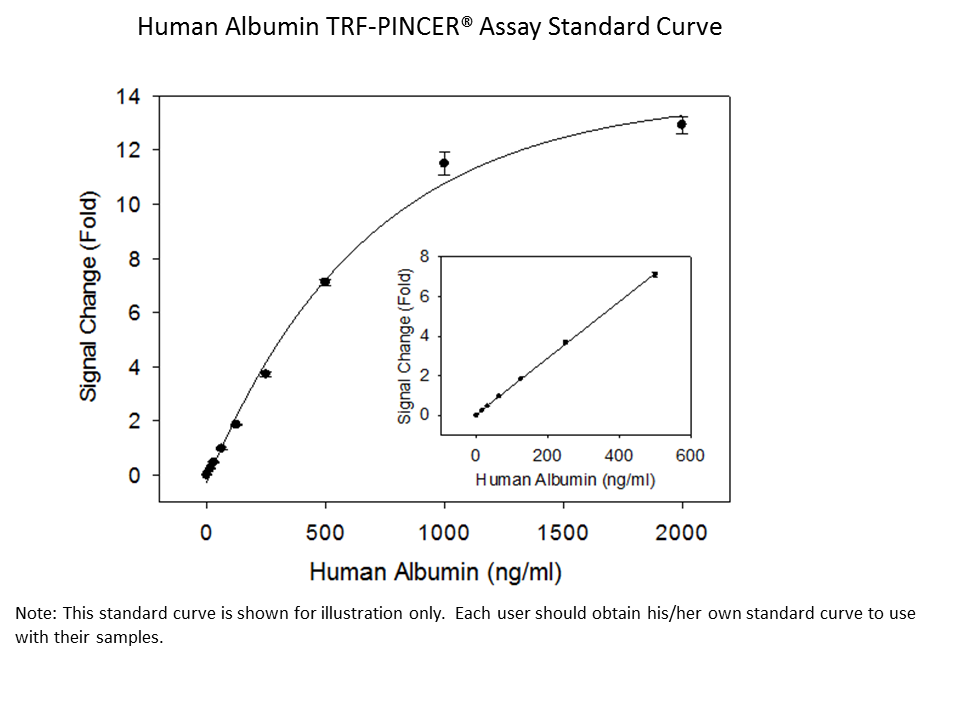
Example of the Standard Curve
Limit of Detection (LOD): 15.6 ng/mL
CV: <5%
Product Features:
-
Easy
Mix test sample or standard with the assay solution and incubate at ~25°C -
Fast
Read fluorescent signal after 30 minutes of incubation -
Sensitive
Assay measures human albumin with an LOD of 15.6 ng/mL. -
Flexible
Assay is adaptable to both low- and high-throughput screening formats
Pricing: The 100-well kit is a one-time trial purchase. For bulk pricing options, please contact us. For any other questions or comments, please do not hesitate to contact us. We are always happy to help!
Please refer to our technology pages for information on the scientific principles and technological advancements of the TRF-PINCER® assays.
Customer Publications using Mediomics’ Human Albumin Pincer Assay Kit:
-
Advancement of analytical modes in a multichannel, microfluidic droplet-based sample chopper employing phase-locked detection. Negou JT, Hu J, Li X, Easley CJ. Anal Methods. 2018 Jul 28;10(28):3436-3443. doi: 10.1039/C8AY00947C. Epub 2018 Jun 5.
-
PINCER® is a registered trademark of Mediomics, LLC, St. Louis, Missouri, U.S.A. Mediomics has a worldwide, exclusive license for this assay platform from Saint Louis University, St. Louis, Missouri.
-
The TRF-PINCER® Assay kit is provided for laboratory R&D use only. This product is not approved by the U.S. Government or by the government of any other country of the world for use in the clinical diagnosis of disease, or treatment of disease in humans or animals.
-
Patents allowed and pending in the U.S. and abroad.
-
PINCER® products have been developed with support, in part, from the National Institutes of Health, through STTR Phase I and Phase II Grants.
Catalog #: AHTR1005-100 , AHTR1005-384
Protocol:
MSDS: