Bridge-It® Cyclic AMP (cAMP) Designer Assay Kit, 96-well format
The Bridge-It® cAMP assay is a highly specific assay to quantify cAMP in stimulated cells. ATP, AMP, and cGMP have all been tested for selectivity using the cAMP assay. No response was detected using the Bridge-It® cAMP assay with any of these compounds within the concentration range that is expected in biological samples (i.e., millimolar range for AMP and ATP, and micromolar range for cGMP).
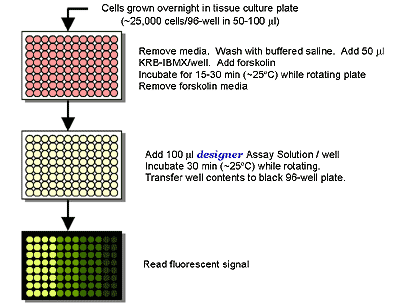
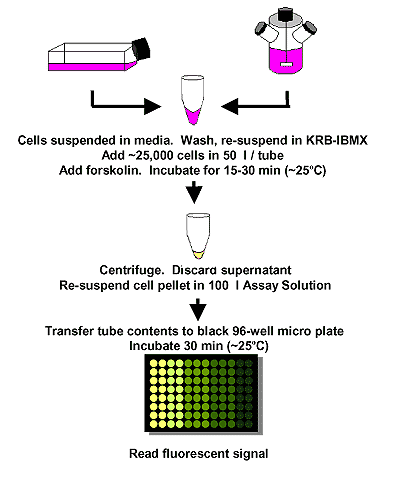
An all-in-one solution is added to activated cells and incubated for 30 minutes. Clarified lysate samples are transferred to a black 96-well microplate to read the fluorescence signal.
$200.00 – $375.00
Description
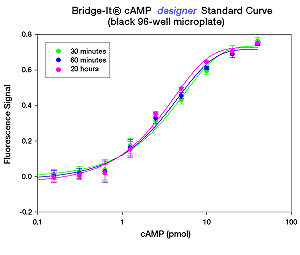
The Bridge-It® Cyclic AMP (cAMP) assay is highly specific. ATP, AMP, and cGMP have all been tested for selectivity using the cAMP assay. No response was detected using the Bridge-It® cAMP assay with any of these compounds within the concentration range that would be expected to occur in “real life” samples (i.e., millimolar range for AMP and ATP, and micromolar range for cGMP). The sensitivity and performance characteristics of the Bridge-It®cAMP designer and the Bridge-It® all in one assay products are presented in the following Table and Figures.
Bridge-It® cAMP Assay |
Microplate Format |
cAMP Detection Level |
designer |
96-well |
5nM (0.5 pmol/well in 100 µl vol.) |
all in one |
384-well |
5nM (100 fmol/well in 20 µl vol) |
Pricing: The 50-well kit is a one-time trial purchase. For bulk pricing options, please contact us. For any other questions or comments, please do not hesitate to contact us. We are always happy to help!
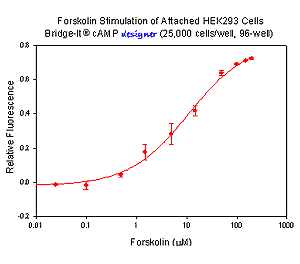
Example of Bridge-It® cAMP designer Assay for Attached Cells
The Bridge-It® cAMP designer assay allows for the stimulation and measurement of cAMP in adherent monolayer cells attached to the wells of a tissue culture microplate. In the following example, HEK 293 cells were trypsinized and plated into the wells of a 96-well polystyrene tissue culture microplate at a density of 25,000 cells per well in 50 µl of culture media. The cells were allowed to attach to the bottom of the wells overnight. The media was removed the next day and the wells were washed with a buffered saline solution. The wash buffer was then removed and replaced with 50 µl of KRB-IBMX buffer. For stimulation, 1 µl of forskolin at different concentrations was added to each well and incubated and rotated gently for 15 minutes at ~25ºC. To quantify cAMP in each well, the forskolin buffer solution was removed, 100 µl of the designer Assay Solution was added to each well, and the microplate was incubated for 30 minutes at ~25ºC with rotation. The well contents were then transferred into a black 96-well microplate and the fluorescent signal was read using the settings for fluorescein (~480 nm excitation, ~520 nm emission).
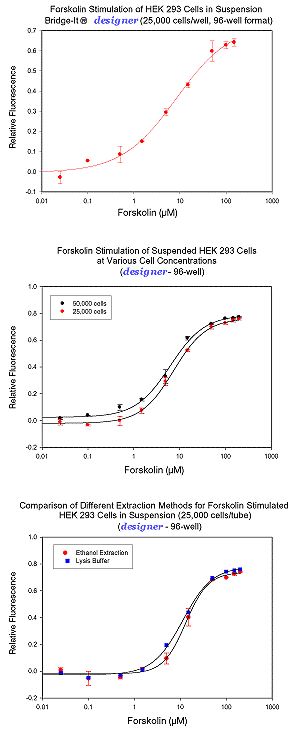
Cell Preparation and Forskolin Stimulation – HEK-293 cells were grown using standard cell culture conditions to ~70-80% confluency. The cells were trypsinized, harvested, and washed in serum-free Krebs Ringers Bicarbonate buffer containing a phosphodiesterase inhibitor (i.e., KRB-IBMX buffer). Cyclic AMP (cAMP) levels were measured following forskolin stimulation of the cells using the Bridge-It® designer assay. Following incubation with Bridge-It® Assay Solution for 30 min at ~ 25°C, the fluorescent signal was read at ~480 nm excitation, ~520 nm emission. Data analysis was performed using relative fluorescence (RF) as a signal change relative to a blank (RF= (F0-F)/F0). RF analysis has been shown to be highly reproducible and does not depend greatly on the instrument used to read fluorescence. Examples can be seen below.
Example of Bridge-It® cAMP designer Assay for Cells in Suspension
For information on the assay principle please see the Bridge-It Assay Technology platform page or download the protocol.
This product is protected by patents and pending patents.
Selected Customer Publications using Mediomics’ Bridge-It cAMP designer Assay Kit:
-
The Microbiota-Produced N-Formyl Peptide fMLF Promotes Obesity-Induced Glucose Intolerance. Wollam J, Riopel M, Xu YJ, Johnson AMF, Ofrecio JM, Ying W, El Ouarrat D, Chan LS, Han AW, Mahmood NA, Ryan CN, Lee YS, Watrous JD, Chordia MD, Pan D, Jain M, Olefsky JM. Diabetes. 2019 Apr 22. pii: db181307.
-
Stimulatory TSH-Receptor Antibodies and Oxidative Stress in Graves Disease. Diana T, Daiber A, Oelze M, Neumann S, Olivo PD, Kanitz M, Stamm P, Kahaly GJ. J Clin Endocrinol Metab. 2018 Oct 1;103(10):3668-3677.
-
Chronic fractalkine administration improves glucose tolerance and pancreatic endocrine function. Riopel M, Seo JB, Bandyopadhyay GK, Li P, Wollam J, Chung H, Jung SR, Murphy A, Wilson M, de Jong R, Patel S, Balakrishna D, Bilakovics J, Fanjul A, Plonowski A, Koh DS, Larson CJ, Olefsky JM, Lee YS. J Clin Invest. 2018 Apr 2;128(4):1458-1470.
-
Toxicity of Cry1A toxins from Bacillus thuringiensis to CF1 cells does not involve activation of adenylate cyclase/PKA signaling pathway. Portugal L, Muñóz-Garay C, Martínez de Castro DL, Soberón M, Bravo A. Insect Biochem Mol Biol. 2017 Jan;80:21-31.
-
Pannexin 3 is required for normal progression of skeletal development in vertebrates. Oh SK, Shin JO, Baek JI, Lee J, Bae JW, Ankamerddy H, Kim MJ, Huh TL, Ryoo ZY, Kim UK, Bok J, Lee KY. FASEB J. 2015 Nov;29(11):4473-84.